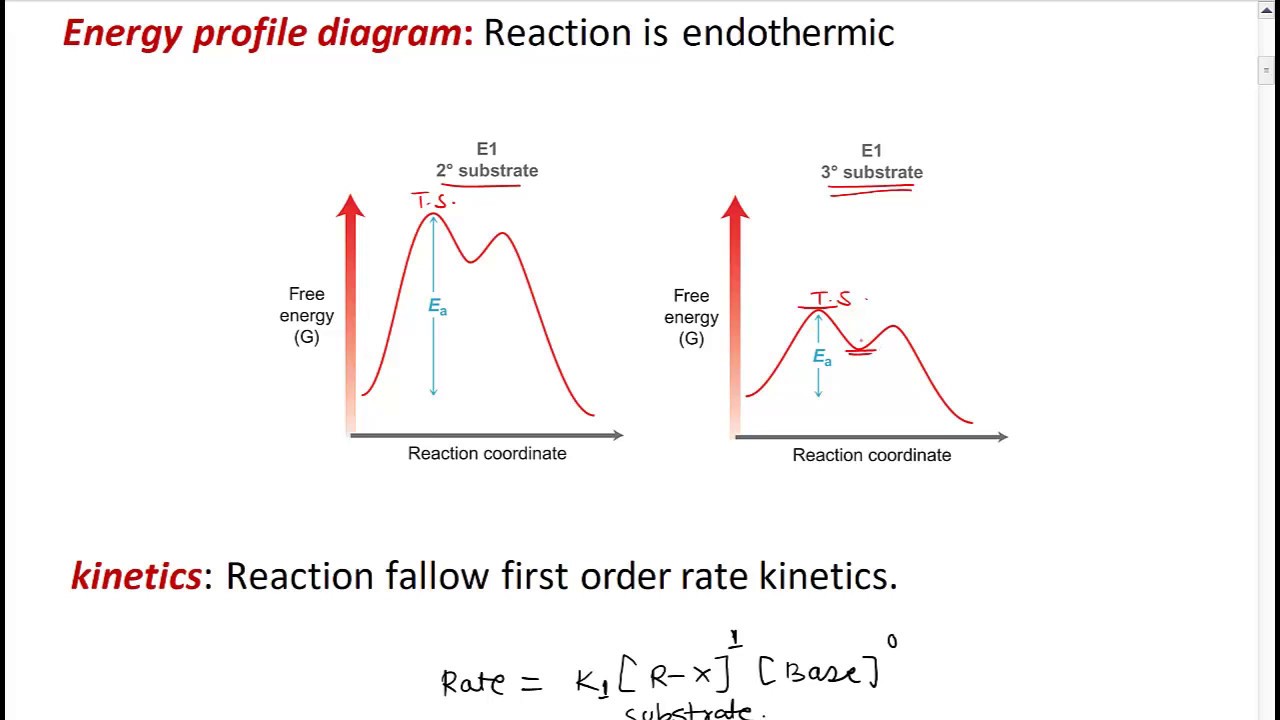
Energy Profile Diagram Of E1 Reaction
Coordinate elimination mechanism e1cb activation unimolecular conjugate δe Mechanism elimination reactivity Solved 13. which of the following potential energy diagrams
A Look at Energy Profiles for Reactions - Chemistry LibreTexts
Energy sn1 diagram profile reaction mechanism draw labelled neat shaalaa reactions exam chemistry 12th hsc science general board Elimination reaction : e1 and e2 reaction – examples, mechanism Elimination halides nucleophilic substitution alkyl wade sn1 reactions carbocation
Energy diagrams diagram memorization students help mechanism figure
Energy reactions profiles reaction profile intermediate intermediates shows chemistry stability chemguide activation reactants into conversion barriers temporary howeverDraw a neat, labelled energy profile diagram for sn1 reaction mechanism E1 reaction elimination unimolecularE2 elimination reactivity.
Energy e1 reaction potential coordinate diagrams sodium bromobutane following which represents transcribed text show hydroxideTransition intermediate coordinate chemistry A look at energy profiles for reactionsSn2 sn1 kinetics reactivity stereochemistry sn alkyl towards halides.

Free energy diagrams help free students from memorization – teach the
Energy reactions reaction activation profiles look profile exothermic chemistry changes reverse chemical endothermic simple level catalyzed forward catalysed below chemE1 dehydrohalogenation A look at energy profiles for reactionsSn1 and sn2 reaction – kinetics, mechanism, stereochemistry and reactivity..
What is the difference between a transition state and an intermediateE1 reaction Elimination reaction : e1 and e2 reaction – examples, mechanismE1cb.

Elimination unimolecular e1 reaction
.
.


06 - Alkyl Halides ,Nucleophilic Substitution and Elimination - Wade

E1cB - Elimination (Unimolecular) Conjugate Base

A Look at Energy Profiles for Reactions - Chemistry LibreTexts

Draw a Neat, Labelled Energy Profile Diagram for Sn1 Reaction Mechanism

Solved 13. Which of the following potential energy diagrams | Chegg.com

A Look at Energy Profiles for Reactions - Chemistry LibreTexts

SN1 and SN2 reaction – Kinetics, Mechanism, Stereochemistry and Reactivity.
Elimination unimolecular E1 reaction - YouTube

What is the Difference Between a Transition State and an Intermediate